Support
Here you can find various information about our products as a supplement to the information on page Products.
The latest software version for the Serial Diluter is available for download under Software.
Instructions
Videos
S
Publications
| Brochure "Inlabtec - Clever Solutions to Serial Dilutions" Other languages: DE | |
||
| Article Clever serial dilution process provides better performance at lower costs, Food Safety Magazine https://www.foodsafetymagazine.com/signature-series/clever-serial-dilution process-provides-better-performance-at-lower-costs/ | ||
| Test results Campden BRI: Method comparison Inlabtec Serial Diluter vs. traditional test tube method. Calculated Correlation Coefficient: 0.998 |
||
| Poster presentation New Taipei Health Department - Comparison of the results of food hygiene standard tests obtained by use of 10:90 and 1:9 serial dilutions. The results showed no statistically significant difference between the bacterial counts obtained from 10:90 serial dilutions or from 1:9 serial dilutions. Original poster in Chinese. |
||
| Poster presentation New Taipei Health Department - Comparison of the results of food hygiene standard tests obtained by use of 10:90 and 1:9 serial dilutions. The results showed no statistically significant difference between the bacterial counts obtained from 10:90 serial dilutions or from 1:9 serial dilutions. Translated from Chinese. |
||
| Fachartikel Beutel statt Reagenzglas - Durch die Verwendung steriler Beutel sowie eine Umstellung im Ablauf können aufwendige Arbeitsschritte und deren Fehlerrisiko eliminiert werden. Austrian Life Science Report 2018.3 | ||
| Fachartikel Step by Step - Wie Teilautomatisierung bei der Probenvorbereitung die Zuverlässigkeit der Keimzahlbestimmung erhöhen kann. Labo 11/ 2017 |
||
| Fachartikel Serial Dilutions in Cost Effective Single-Use Sterile Bags - Verifications by method comparison confirm the equivalence of the new method which relieves staff and increases the productivity of the testing laboratory. Lifesciences plus 02/ 2015 | ||
| Fachartikel Serielle Verdünnung leicht gemacht - Mit den Inlabtec Serial Dilution Bags bleibt die Einfachheit und Zuverlässigkeit erhalten, während der Zeit- und Ressourcenaufwand massiv reduziert wird. Lebensmittel-Technologie 3/ 14 | ||
| Testbericht Amt für Verbraucherschutz AG: Untersuchung zur Wirksamkeit der Inlabtec Pipette Filter Plugs - Die Ergebnisse der Sterilkontrollen demonstrieren, dass die geprüften iNLABTEC Filter ungewollte Kontaminationen wirkungsvoll unterbinden. |
||
Software
| Software Serial Diluter Version AP240628 as ZIP file. After extraction transfer file "bag.mhx" on a USB stick (>version 3.0) for the software update. |
||
Questions and Answers
1. Introduction and Benefit
What is the Serial Diluter, and for which laboratories is it intended?
The Serial Diluter is designed for users performing dilutions for the plate count method in microbiological testing of food, feed, cosmetica, etc.
What do I save in costs and time using the Serial Diluter?
By using the Serial Diluter, you can save both time and costs on your dilution workflow while also promoting greater ecological sustainability.
The system allows for faster dilutions by eliminating the need for manual vortexing or handling of tubes and caps. This can save you up to 3 – 5 seconds of the time required for manual dilutions and result in significant time savings depending on your specific workflow.
Additionally, the Serial Diluter can save you up to 50 cents per dilution step due to completely eliminating the need for preparing test tubes or using expensive pre-filled dilution tubes, which results in substantial plastic waste reduction.
You’ll also save on associated costs such as water, electricity, and cleaning agents while further reducing your laboratory’s environmental footprint.
Overall, the Serial Diluter can reduce your consumables costs by up to 80 % depending on your actual cost structure.
Finally, because the Serial Diluter reduces the need for transporting, storing, and disposing of consumables, it further results in major cost savings associated with waste disposal and other related costs, contributing to a more sustainable future.
Does the Serial Diluter improve the reliability and accuracy of test results?
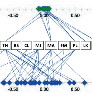
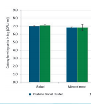
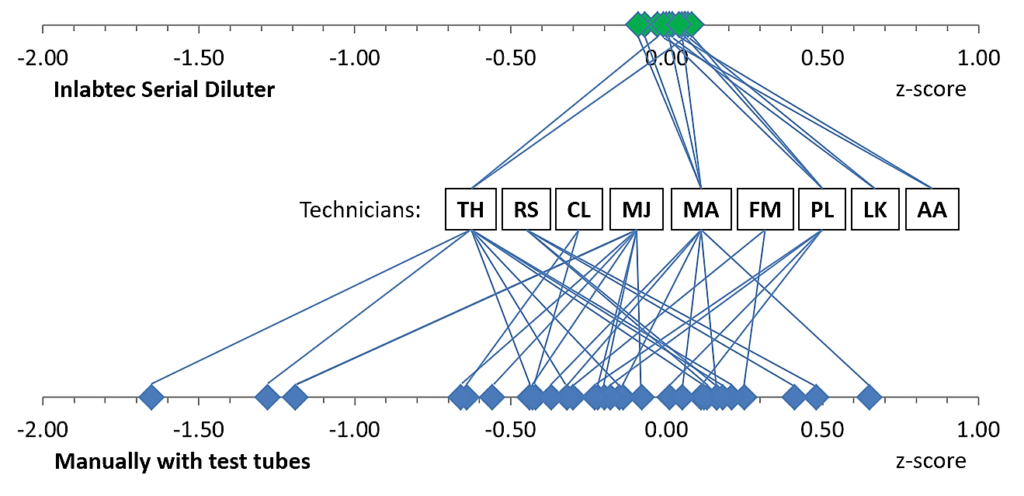
The Serial Diluter ensures the accuracy of testing results, regardless of sample throughput, time pressure, unplanned work peaks, employee experience, and other factors. Moreover, evaluation of interlaboratory tests shows that all employees using the Serial Diluter achieve z-score values of ± 0.1 (see Figure below). This means that regardless of their level of experience or individual differences in working technique, all employees delivered almost identical test results.
What are the benefits of automating dilution procedures in the laboratory?
The Serial Diluter offers efficiency improvements by automating labor-intensive tasks, allowing operators to focus on other responsibilities, and ultimately creating a more positive working environment for laboratory technicians.
What sample throughput is possible per Serial Diluter?
The table shows the time needed in minutes for a certain number of dilutions and inoculations of culture media.
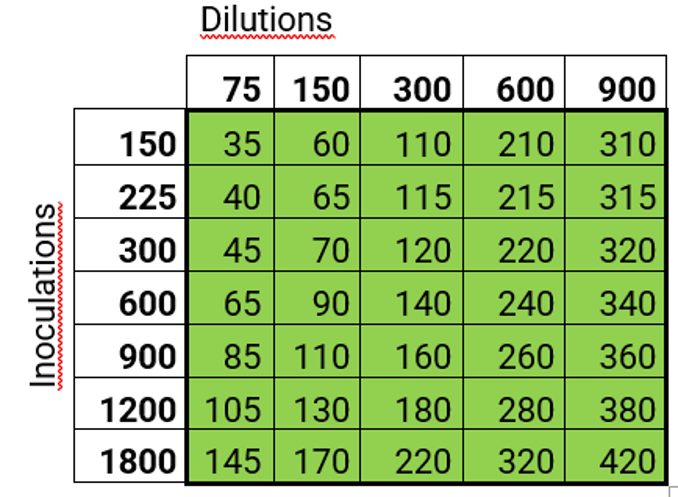
Example: The time required for 100 samples with 3 dilutions per sample (total 300 dilutions) and 2 inoculations per dilution (total 600 inoculations) is 140 minutes. Calculation basis: Average 10 seconds per dilution including bag insertion and removal, 4 seconds per inoculation of Petri dishes, 30 seconds per sample for placing the plates on the table for the inoculations.
For what sample throughput is the Serial Diluter worth using?
Depending on the costs and the available resources, the use is worthwhile from 100 dilutions per day, i.e. about 30 samples at 3 dilutions each.
The payback time is determined by the previous cost of preparing test tubes or the cost of pre-filled disposable dilution vials. On Our Solution a savings calculator can be found to check whether the purchase of a Serial Diluter is worthwhile for your laboratory under the given circumstances.
Is the instrument also suitable for samples that require only 1 or 2 dilutions?
For labs dealing with lots of samples, worrying about how many dilutions each sample gets isn’t a big deal. For example, if you load six bags, the Serial Diluter can handle up to six samples with one dilution or three samples with two dilutions all at the same time. This saves time and boosts productivity, which is great for labs dealing with heaps of samples every day.
How much storage space do I save with Serial Dilution Bags?
A box Serial Dilution Bags 100101 (2’000 bags) has a size of 13 cm x 16 cm x 12.5 cm and requires a storage volume of approximately 2.4 l.
Compared to the same amount of filled test tubes or cups, the need for storage space is about thirty times less.
How does the Inlabtec Serial Diluter compare to other microbial dilution methods?
There are several methods available for preparing dilutions in the laboratory, each with its own advantages and disadvantages. Some commonly used methods include test tubes prepared by the laboratory, ready-made disposable dilution vials, the spiral plater, and the Tempo MPN system.
While test tubes prepared by the laboratory are an established standard method that most laboratory technicians are familiar with, they can be time-consuming to prepare and require manual skill and good workplace organization to be efficient and yield correct cell counts.
Ready-made disposable dilution vials may be immediately available and require no preparation, but they come with significant costs for procurement, transport, storage, and disposal, as well as a high environmental impact. Additionally, not all diluents may be available, and carrying out dilutions is not always simplified and accelerated.
The spiral plater is well-suited for certain applications without having to dilute the intial sample, such as beverages and milk, and can yield up to 105 CFU/ml and plate. However, it is sensitive to clogging by particles in the sample, requires a high level of flatness for the culture media, and does not support cast plates or dry film media such as Petrifilm or Compact Dry. Additionally, a special counting procedure is necessary for colonies on the plates, and regular maintenance and servicing are necessary.
The Tempo MPN system is a fully automated system for microbial count that is ideal for very high throughput and uniform sample types and often manages without additional dilutions. However, it is less accurate than the standard method, and no visual control is possible. It also comes with high operating and acquisition costs, requires significant validation effort per sample type, has a high environmental impact, and requires significant maintenance and service costs. Mention that, in many cases, samples must be diluted 1:100 before use, and this dilution is done with the Serial Diluter.
In comparison, the Inlabtec Serial Diluter presents a streamlined and automated approach to dilution preparation, combining efficiency with precision. By eliminating inaccurately filled test tubes and the need for vortexing, it mitigates the risk of inhomogeneous mixtures, ensuring consistently accurate analysis results. The system’s high flexibility allows it to accommodate various diluents, sample types, and plates. Moreover, the Serial Diluter stands out as an environmentally friendly alternative to disposable dilution vials. Its automated process not only saves time but also enhances productivity, making it advantageous even for laboratories with lower daily sample volumes.
2. Technical Information
What is simplified, is different?
- Eliminates the time-consuming preparation of test tubes..
- Eliminates the need to purchase expensive disposable test tubes.
- Accelerates dilution through automation, improving overall efficiency.
- Simplifies handling, reducing the risk of repetitive strain injuries, as automatic mixing eliminates the need to manipulate test tubes and lids manually.
Can I use the Serial Diluter without any training?
The installation process is straightforward, resembling that of a gravimetric diluter. Our comprehensive support, including training videos, an illustrated manual, and a quick start guide, ensures a smooth transition to the Serial Diluter. While adopting the Inlabtec Serial Diluter may involve a brief adjustment period, the investment proves worthwhile.
How is the diluent dispensed?
The Inlabtec Serial Diluter resembles a pipetting aid. It utilizes vacuum for liquid aspiration and pressure for depositing it into a Serial Dilution Bag. An optical sensor, positioned sideways on the pipette at the required volume, ensures precision by eliminating the need for user judgment, in contrast to the requirements of a typical pipetting aid. The system is regulated by pinch valves for consistent results and never comes into contact with the liquid. Tubings and Dispensing Nozzle can be easily removed for cleaning and sterilization.
Which serological pipettes and graduated pipettes can be used with the Serial Diluter?
The Inlabtec Serial Diluter is compatible with standard 10 ml serological and graduated pipettes, whether made of glass or plastic. It can also accommodate other pipette sizes, as long as their outer diameter falls within the range of 8-15 mm, and their length is between 150-350 mm.
It’s important to note that wider-opening serological pipettes commonly used in cell culture work should be avoided, as they may not securely connect to the Serial Diluter.
How accurate are 10 ml pipettes?
The indicated volume inaccuracy for plastic serological pipettes is approximately ± 2%, while for glass graduated pipettes, it is ± 0.5% for class AS and ± 1% for class B.
How accurate can be dispensed with the Serial Diluter?
The volume in the Serial Diluter is determined by a pipette in conjunction with an optical sensor. The precision of the dispensed volume depends on the accuracy of the pipette’s scale/graduation and the dispensed dead volume associated with the pipette connection at the Serial Diluter. This dispensed dead volume is constant and is affected by the compatibility between the pipette outlet and the pipette connection and typically falls within the range of 50 – 100 µl.
How to perform a pipette scale calibration?
To ensure accurate and consistent results, it is recommended to perform a quick check of the 10 ml pipettes in use. This check, also known as pipette scale calibration, helps determine the systematic deviation of the pipette scale and establish the correct sensor position for the desired volume of 9 ml for serial dilutions. Calibration is easy to perform with a balance and can be done quickly with instructions available under our Support section. Once calibrated, all pipettes within the same lot number will have the same settings, simplifying the process of setting the correct volume during pipette installation.
How can a shortend pipette check be performed?
For a quick check, detach the silicone connector from Tubing Set 100011 and affix it to the pipette outlet. Use a balance or graduated cylinder along with a pipetting aid to determine at which point on the pipette scale the desired 9 ml are pipetted with the silicone connector attached. Record this value and use it for pipettes with identical lot numbers when positioning the optical sensor of the Serial Diluter.
How can I be sure that the set volume was actually dispensed?
The volume can be visually checked before dispensing by reading the meniscus position in the pipette. To verify the volume defined by the sensor position, either dispense into a graduated cylinder or weigh a filled bag.
What is the best way to pipette into a measuring cylinder for verification?
To pipette into a measuring cylinder, remove the Dispensing Nozzle from the Bag Holder and position it above a 10 ml measuring cylinder. Trigger dispensing by moving the dosing arm of the Bagholder to the next position. To reduce the risk of contamination during verification, consider attaching a dispensing nozzle holder 140015 to the Serial Diluter (see picture below).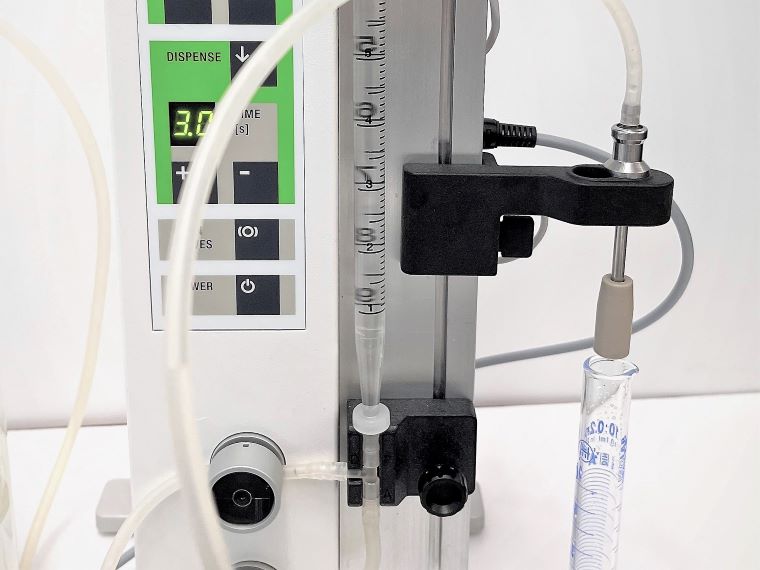
What is the difference between accuracy and precision?
Accuracy indicates how close the average actual value, i.e. the mean value, is to the target value. The deviation is also called systematic error. The precision indicates how close the individual measured values are to each other. The statistical measure of precision is the standard deviation, often called random error.
How to assess mixing efficiency?
To assess mixing efficiency, a rapid check of the mixing process—whether conducted manually with test tubes or automatically with the Serial Diluter—can be performed by measuring turbidity. Instructions, along with an Excel template, are available under Support/Instructions.
Can I leave the Serial Diluter operational overnight and continue working the next day?
One of the great advantages of the Inlabtec Serial Diluter is that it can remain operational until the connected diluent is used up. This feature allows laboratories to save time and increase productivity by avoiding interruptions caused by reconnecting diluents. Additionally, the Serial Diluter is designed with a protective sleeve that shields the dispensing nozzle from laboratory air, minimizing the risk of contamination and ensuring accurate results.
To further ensure the accuracy of results, the Serial Diluter has a dosing tip that can be parked in 70% ethanol when not in use for a longer period of time, such as over the weekend. This additional precautionary measure helps maintain the cleanliness of the system and prevent potential contamination. For more details on how to properly park the dosing tip, please refer to the operating instructions in support.
Can the system be operated in the sterile bench and UV-sterilized overnight?
The Serial Diluter can be operated in a sterial bench, laminar flow, etc. and the device is UV-resistant for regular surface sterilisation.
How reliable works the opening of the Serial Dilution Bags? Is there wear and tear?
A microstructured silicone is used to open the Serial Dilution Bags wear-free. In the event that the adhesive force reduces due to dirt, the adhesion gripper can be easily cleaned with water, 70% ethanol or other appropriate cleaning solutions, restoring it to its original functionality. However, if cleaning does not restore the adhesive force and the gripper has been in use for 6-12 months, it may be time to replace it. Don’t worry, replacing the gripper is easy, and we have training videos available to guide you through the process.
How do I clean the Serial Diluter?
To clean the device surfaces, use a commercially available cleaner or soapy water, and for disinfection, use 70% ethanol.
To ensure reliable and consistent results, it is crucial to properly clean and sterilize the liquid-conducting parts of the Serial Diluter. Replace the diluent with water after use and rinse the system before removing the hoses and dosing tip. For more detailed instructions, please refer to the operating manual or training videos available on our website..
Are there components that need to be replaced regularly?
To maintain the highest level of sterility and accuracy, it is recommended to replace the sterile filters in the diluting solution bottles after every eight autoclaving cycles, as recommended by the filter manufacturer. In addition, it is advised to change the sterile filter on the unit every 6 – 9 months to ensure optimal performance and prevent potential contamination. Proper filter maintenance and replacement can help ensure reliable and consistent results in your laboratory work.
What kind of materials does the diluent come into contact with?
The diluent comes into contact with silicone, stainless steel, glass or polystyrene (depending on the 10 ml pipette used), polyethylene and polypropylene.
What maintenance and service is required for the Serial Diluter?
Experience effortless operation with the Serial Diluter – no regular maintenance or servicing required! The device automatically detects any issues, such as clogged filters or leaks, and alerts you immediately. Simply change the accessible filters and adhesion grippers for hassle-free maintenance. And in the unlikely event that something still isn’t quite right, our expert sales and service partners are always on hand to assist you.
How is the service of the products ensured?
Inlabtec products are sold through authorised distributors, who can perform all service work. The Serial Diluter is based on very solid, proven technology, which keeps service work to an absolute minimum.
How long is the warranty period of the Serial Diluter?
The warranty period for all device components is two years. Details can be found in our AGB (General Terms and Conditions).
3. Serial Dilution Bags
Using Serial Dilution Bags, what are the cost savings per dilution?
The costs per dilution are reduced on average by a factor of 3 – 5 if readymade disposable dilution vials or cups have been used up to now.
If the test tubes are still washed, filled and sterilized in the laboratory, the costs per dilution are reduced depending on the saved working time and its costs as well as the reduced infrastructure costs.
Savings and payback time is determined by the previous cost of preparing test tubes or the cost of pre-filled disposable dilution vials. On Our Solution a savings calculator can be found to check whether the use of a Serial Diluter is worthwhile for your laboratory under the given circumstances.
Is it ecologically sensible to use Serial Dilution Bags?
Serial Dilution Bags reduce plastic waste by a factor of 10-30.
Approximately 250 mg of polyethylene (PE) is required for the production of a Serial Dilution Bag. In contrast, a prefilled disposable container consumes between 2.5 – 7.5 g of plastic, depending on the design. One kilogram of polyethlyene is sufficient for 4’000 dilutions in Serial Dilution Bags; with conventional consumables, it is only sufficient for 130 to max. 400 dilutions.
The energy consumption for transport is also lower by factors: 250 mg per serial dilution bag vs. 11.5 – 16 g per plastic container filled with 9 ml diluent. It is questionable whether it makes sense to send saline/peptone water around the world, especially since the diluent for primary dilution in the stomacher is already available in the laboratory and the quantity can be easily increased.
The energy consumption for the production of the Serial Dilution Bags is also moderate compared to the rinsing of test tubes: The energy consumption of one rinsing machine cycle corresponds approximately to the consumption for the production of 400 Serial Dilution Bags. Together with the energy required for filling and autoclaving the test tubes, the expected additional demand for the bags is not too great, if at all.
What is the energy consumption for the production of Serial Dilution Bags?
The energy required to manufacture 1’000 Serial Dilution Bags is approximately 20 MJ. Of this, 8 MJ (40%) is recovered in waste incineration. The net energy requirement is therefore around 12 MJ for 1’000 Serial Dilution Bags.
For comparison: a washing cycle for 1’000 test tubes with a dishwasher consumes about 4 – 6 MJ of energy and sterilization in a steam autoclave consumes about 8 – 10 MJ.
Can Serial Dilution Bags be recycled?
The used Serial Dilution Bags can be recycled with other polyethylen waste (PE). In this case, the health risks due to the potential contamination of the bags by the samples tested must be taken into account and appropriate precautions must be taken.
We believe that eliminating plastic waste as far as possible and reasonable is the best strategy to protect our environment. This principle was the driver for the development of the Serial Diluter.
What material are the Serial Dilution Bags made of?
The Serial Dilution Bags are made of high purity virgin polyethylene (PE) under clean room conditions. The Serial Dilution Bags comply with the regulations EU No. 10/ 2011 of 14.1.2011, 1935/ 2004 of 27.10.2004 and 2013/ 2006 of 22.12. 2006 and U.S. FDA 21, CFF 04, Title 21 (plastic materials and articles in contact with food).
Shelf life of the Serial Dilution Bags?
The shelf life at room temperature of the Serial Dilution Bags is guaranteed for five years after production, provided the box is shrink-wrapped in the protective film.
How long can an opened box of Serial Dilution Bags be used?
After removing the protective film from the packaging it is recommended to use the Serial Dilution Bags within three months.
What are the optimal storage conditions for the Serial Dilution Bags?
Store protected from direct solar light at room temperature (10 – 30 °C).
Can I store the filled Serial Dilution Bags?
The used Serial Dilution Bags can be stored in the Bagshell 100030. A Bagshell is included in the scope of delivery.
What is the evaporation rate with filled and stored Serial Dilution Bags compared to classical dilution tubes?
The evaporation rate for filled Serial Dilution Bags and dilution tubes in the refrigerator is identical with approx. 5 – 10 mg (µl) per 24 hours per bag or capped test tube with 9 ml of diluent.
Do the Serial Dilution Bags contain any substances that could inhibit bacterial growth?
As the Serial Dilution Bags are made of high-purity virgin polyethylene and free of any substances that could inhibit the growth of microorganisms.
How is the sterility of the Serial Dilution Bags ensured?
The Serial Dilution Bags are produced under strict clean conditions that comply with DIN EN ISO 13485 regulations for medical device manufacturers. In addition, the boxes are radiation sterilized according to ISO 11137-1/-2 standards to ensure optimal sterilization. The sterility of the Serial Dilution Bags is validated by an independent test laboratory through dosimetric and microbiological testing, which has confirmed the absence of microbial growth and the sterile nature of the bags. Our commitment to quality is reflected in our continuous monitoring of the sterility of the Serial Dilution Bags, which adhere to a high Sterility Assurance Level (SAL) of 10-3 .
How is a homogeneous mixing in the Serial Dilution Bags ensured?
The Serial Dilution Bags have been developed together with the Serial Diluter TA for homogeneous dilutions and are characterised as follows:
1. The Serial Dilution Bags are divided in the Serial Diluter into an addition and removal zone in order to exclude carry-over with higher concentrated sample material
2. 45° bevels on the bottom ensure a targeted deflection of the incoming liquid jet, creating a perfect counter-clockwise vortex.
3. The liquid jet is accelerated by an additionally generated pressure surge to quickly form an intensive vortex.
4. The rising air at the end of the liquid delivery causes an abrupt reversal of the vortex’s direction of rotation.
The Serial Diluter UA has an integrated mixer which ensures homogeneous mixing of sample and diluent in the Serial Dilutioni Bags triggered by simply moving the handle from one position to the next. See demo video.
4. Compliance and Certification
Which directives and ISO standards were taken into account?
Serial dilution with the Serial Diluter complies with the standard method according to ISO 6887-1:2017 Microbiology of the food chain – Preparation of test samples, initial suspension and decimal dilutions for microbiological examination – Part 1: General rules for the preparation of the initial suspension and
decimal dilutions and the associated normative reference ISO 7218:2007.
How was compliance with the ISO standards checked during development?
The serial dilution with the Serial Diluter was verified against the standard technique with dilution tubes according to ISO 6887-1 by the Zurich University of Applied Sciences (ZHAW Wädenswil). In addition, the Serial Diluter was tested by ISO 17025 accredited users of the Serial Diluter on the basis of direct comparisons with the test tube technique using various food samples (see test results Campden BRI).
Do instructions and a template exist for checking the Serial Diluter in accordance with ISO 6887-1?
Yes, the operating instructions outline how to check the dispensed volume and verify whether the accuracy meets ISO 6887-1 standards. Both the operating instructions and the Excel template can be accessed under Support/ Instructions.
An ISO 6887-1 approach to assess mixing efficiency —whether conducted manually with test tubes or automatically with the Serial Diluter—can be performed by measuring turbidity. Instructions, along with an Excel template, are available under Support/Instructions.
What is the difference between verification and validation of a testing method?
Method validation and method verification are distinct but equally important processes used to ensure the reliability of testing methods. Method validation is conducted when a laboratory develops a new testing method. It involves evaluating whether the method is suitable for its intended purpose and meets required performance criteria, such as accuracy, precision, and sensitivity.
In contrast, method verification is used to confirm that a previously validated or standardized method performs as expected in a specific laboratory setting. This process is necessary when a laboratory uses a method for the first time or when modifications to the method could potentially impact its performance.
While both processes aim to ensure accurate and reliable results, validation is for new methods, whereas verification ensures the proper implementation of existing methods under specific laboratory conditions.
Do I need to verify or validate the Serial Diluter?
The process for determining microbial counts remains compliant with the standard method outlined in ISO 6887-1:2017 when using the Serial Diluter. However, the device introduces two key differences: it uses bags instead of test tubes and employs mixing based on the Stomacher principle. Consequently, verifying the proper use of the Serial Diluter within the specific laboratory setting is essential before its initial use. This verification process, known as method verification, ensures the Serial Diluter performs as intended and produces accurate, reliable results. This requirement aligns with accreditation standards such as ISO/IEC 17025, which mandate that laboratories demonstrate their competence and ability to produce valid results.
How can I verify the Serial Diluter?
To verify the effectiveness of the Serial Diluter, microbial counts obtained using the Serial Diluter should be compared with those obtained using the sample dilution technique previously employed in the laboratory. The bacterial count determinations from the same food sample, using both dilution methods, should not differ by more than ±0.5 log CFU (colony-forming units). This acceptable variance is based on experience from proficiency tests (interlaboratory comparisons) and is widely recognized as a reliable benchmark for microbial count determination in food samples using the plate method. By comparing results from the Serial Diluter with those from the established method, the laboratory can confirm that the new method produces accurate and reliable results.
Is there a quality certificate for the Serial Diluter?
The CE-certificate for the Serial Diluter is in the manual.
How is the sterility of the system ensured?
The Serial Diluter has several features designed to maintain sterility during operation. All parts of the system that come into contact with liquid, including tubes, the dispensing tip, and the dilution solution, can be steam sterilized. This process ensures that all potential contaminants are eliminated prior to use.
Additionally, the system is designed to only dispense liquid via the dispensing tip, and not aspirate it, which prevents any potential contaminants from being drawn into the system. The system is also ventilated via sterile filters during operation, which provides an effective barrier to prevent the entry of microorganisms.
Furthermore, the operation of the Serial Diluter is specifically designed to eliminate contamination of the dispensing tip during routine use. This is an important feature that helps maintain sterility throughout the entire operation.
Collectively, these features make the Serial Diluter an effective and reliable tool for accurately determining microbial counts while maintaining a sterile environment.
A demo video for the Serial Diluter UA shows the comprehensive safety precautions.
Why can I be sure that the dispensing nozzle will not become contaminated during operation?
The Serial Diluter is designed with multiple safeguards to prevent contamination of the dispensing nozzle, making it a reliable tool for accurate microbial counts. However, it’s important to note that there may always be some level of risk for contamination, no matter which method is used.
To minimize the risk of contamination, the dosing arm moves only in one direction, ensuring that the dispensing nozzle remains in a sterile zone and does not come into contact with unsterile sample material. When filling a Serial Dilution Bag, the dispensing nozzle is inserted into a sterile, empty bag, and the sample is pipetted into the bag through a separate opening, preventing contact and/or contamination of the dispensing nozzle.
Mixing of the sample does not start until the dispensing nozzle is out of the bag and already above the next sterile bag, which eliminates the risk of contamination from the contents of the bag.
In addition, the protective sleeve outside of the bag further minimizes the risk of contamination of the dispensing tip by preventing contact with non-sterile surfaces.
However, it’s important to follow proper procedures and regularly monitor and validate the performance of the system to ensure that accurate results are obtained and the risk of contamination is minimized as much as possible. Environmental factors, such as air quality or temperature fluctuations, may affect the risk of contamination. Therefore, it’s important to store and operate the Serial Diluter in a controlled environment, and to regularly inspect and maintain the components to ensure they are not damaged or compromised.
By following proper procedures and taking appropriate precautions, the risk of contamination with the Serial Diluter can be minimized, ensuring reliable and accurate results for microbial counts.
A demo video for the Serial Diluter UA shows the comprehensive safety precautions.
How can we ensure the sterility of the Serial Diluter in our lab?
The Serial Diluter is designed with comprehensive safety precautions to ensure that it is a reliable and safe tool for accurate microbial counts. The commissioning process is sterile and free from contamination risks, and a training video is available to guide users (see Support).
It’s recommended to use sterilization pouches with a paper layer to ensure complete penetration of steam and reliable sterilization of the Tubing Set and Dispensing Nozzle. Proper precautions must also be taken during installation to ensure that the Dispensing Nozzle does not come into contact with non-sterile surfaces.
It’s is important to note that errors may still occur during installation or sterilization, which could compromise the sterility of the system. However, with experience and adherence to recommended guidelines, the commissioning process can be carried out smoothly and without any problems.
How can I test whether the components used were sterile and the determined bacterial counts are correct?
While sterility cannot be tested in a rapid time, it is still possible to ensure the Serial Diluter is reliable. If you have doubts about the system’s sterility, we recommend performing a sterility check before and after each use. To do this, simply plate an aliquot of the first and last 9 ml diluent dispensed on a culture medium and incubate it for sterility control. This quick and simple check will allow you to confirm that maintaining system sterility is not an issue.
Are the sterile filters used hydrophobic or hydrophilic and are they autoclavable?
The PTFE (Teflon) filters used are hydrophobic and autoclavable. Difference between hydrophobic and hydrophylic filters: Trainings video
Attention: Do not install hydrophilic filters on bottles with diluent or on the Serial Diluter.
5. Environmental Impact
How does the environment benefit from the use of the Serial Diluter??
Serial Dilution Bags offer significant environmental advantages over conventional consumables. By reducing plastic waste by a factor of 10-30, they are a sustainable and cost-effective alternative for microbial testing.
Only 250 mg of polyethylene (PE) is required for the production of one Serial Dilution Bag, whereas a prefilled disposable container consumes between 2.5 – 7.5 g of plastic. This means that one kilogram of polyethlyene is sufficient for 4,000 dilutions in Serial Dilution Bags, compared to only 130 to max. 400 dilutions with conventional consumables.
In addition, the energy consumption for transport is much lower for Serial Dilution Bags, with only 250 mg per bag compared to 11.5 – 16 g per plastic container filled with 9 ml diluent. This makes sense as it’s unnecessary to transport dilution vials filled with saline/peptone water around the world when the diluent is already prepared in larger quantities in bottles for primary dilutions.
Furthermore, Serial Dilution Bags are a more environmentally friendly alternative to conventional consumables as they significantly reduce plastic waste and their energy consumption is comparable, if not more efficient, than traditional methods such as rinsing test tubes. In fact, the energy required for one rinsing machine cycle is estimated to be equivalent to the energy needed for the production of 500 to 1000 Serial Dilution Bags. Additionally, taking into account the energy required for filling and autoclaving dilution vials, Serial Dilution Bags may even be more energy-efficient than conventional consumables.
In conclusion, the use of Serial Dilution Bags not only offers cost-effective and accurate microbial testing but also helps reduce plastic waste and energy consumption, making it an environmentally friendly choice for laboratories.
Can Serial Dilution Bags be recycled??
The Serial Dilution Bags can be recycled with other polyethylene waste (PE). However, it is essential to consider the potential contamination of the used bags and take appropriate precautions to minimize health risks.
At the same time, we strongly believe that reducing plastic waste should be a priority in our efforts to protect the environment. This principle was the driving force behind the development of the Serial Diluter, which significantly reduces plastic waste compared to conventional consumables. By using Serial Dilution Bags, we can not only achieve reliable and accurate results but also minimize our environmental footprint.
If you need additional information, please contact us via contact form. We are happy to be of service.